Our Clinical Research
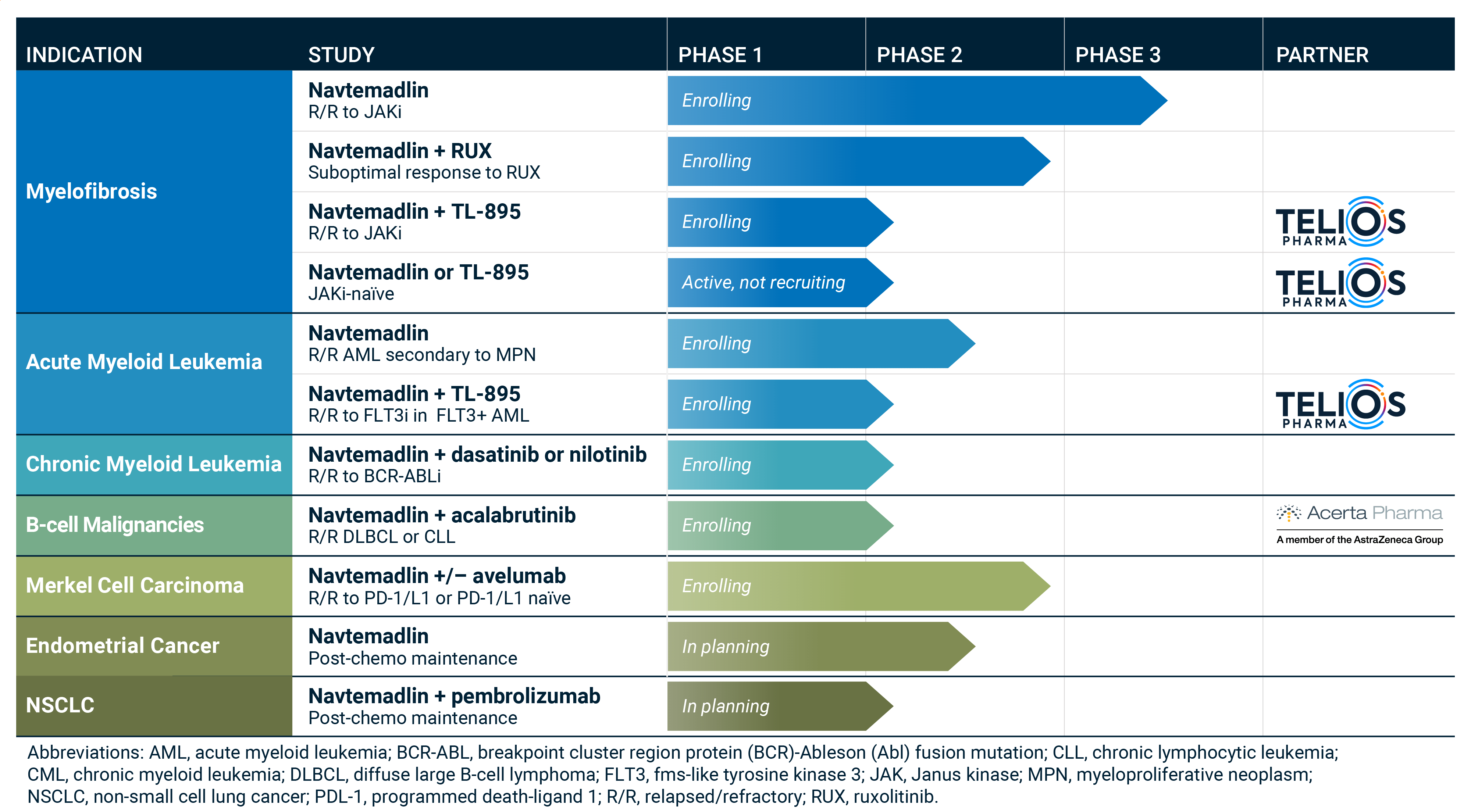

Myelofibrosis (MF) - BOREAS
Status: Recruiting
http://boreas-trial.com (NCT03662126)KRT-232 Versus Best Available Therapy for the Treatment of Subjects With Myelofibrosis Who Are Relapsed or Refractory to JAK Inhibitor Treatment (BOREAS)
Myelofibrosis (MF)
Status: Recruiting
ClinicalTrials.gov: NCT04485260An Open-Label, Multicenter, Phase 1b/2 Study of the Safety and Efficacy of KRT-232 Combined With Ruxolitinib in Patients With Primary Myelofibrosis (PMF), Post-Polycythemia Vera MF (Post-PV-MF), Or Post-Essential Thrombocythemia MF (Post ET-MF) Who Have a Suboptimal Response to Ruxolitinib.
Acute Myeloid Leukemia (AML)
Status: Recruiting
ClinicalTrials.gov: NCT04113616An Open-Label, Multicenter, Phase 1b/2 Study of the Safety and Efficacy of KRT-232 Combined with Low-Dose Cytarabine (LDAC) or Decitabine in Patients with Acute Myeloid Leukemia (AML).
B-cell Malignancies (DLBCL or CLL)
Status: Recruiting
ClinicalTrials.gov: NCT04502394An Open-label, Phase 1b/2 Study of KRT-232 in combination with acalabrutinib in Subjects with B-cell Non-Hodgkin Lymphoma.
Chronic Myelogenous Leukemia (CML)
Status: Recruiting
ClinicalTrials.gov: NCT04835584An Open-Label, Multicenter, Phase 1b/2 Study of the Safety and Efficacy of KRT-232 Combined with a Tyrosine Kinase Inhibitor (TKI) in Patients with Relapsed or Refractory Ph+ Chronic Myeloid Leukemia (CML)
Merkel Cell Carcinoma (MCC)
Status: Recruiting
ClinicalTrials.gov: NCT003787602A Phase 2, Open-Label, Single-Arm Study of KRT-232 in Patients With p53 Wild-Type (p53WT) Merkel Cell Carcinoma (MCC) Who Have Failed Anti-PD-1 or Anti-PD-L1 Immunotherapy.
Endometrial Cancer
Status: Not Yet Recruiting
A Two-part, Randomized Phase 2a/2b Study of Navtemadlin in Subjects with TP53WT Advanced or Recurrent Endometrial Cancer Who Responded after Chemotherapy
Non-Small Cell Lung Cancer (NSCLC)
Status: Not Yet Recruiting
An Open-Label, Multicenter, Phase 1b Study of the Safety, Efficacy and Pharmacokinetics of Navtemadlin plus Pembrolizumab as Maintenance Therapy in Subjects with Locally Advanced and Metastatic Non-Small Cell Lung Cancer
Myelofibrosis (MF)
Status: Recruiting
ClinicalTrials.gov: NCT04640532An Open-Label, Multicenter, Phase 1b/2 Study of the Safety and Efficacy of KRT-232 in Combination With TL-895 for the Treatment of Relapsed or Refractory Myelofibrosis and KRT-232 for the Treatment of JAK Inhibitor Intolerant Myelofibrosis
Myelofibrosis (MF)
Status: Active, Not Recruiting
ClinicalTrials.gov: NCT04878003An Open-Label, Multicenter, Phase 2 Study Assessing the Safety and Efficacy of KRT-232 or TL-895 in Janus-associated Kinase Inhibitor Treatment-Naïve Myelofibrosis
Acute Myeloid Leukemia (AML)
Status: Recruiting
ClinicalTrials.gov: NCT04669067An Open-Label, Multicenter, Phase 1b/2 Study of the Safety and Efficacy of TL-895 Combined with KRT-232 in Subjects with Relapsed/Refractory (R/R) FLT3+ Acute Myeloid Leukemia (AML)
Acute Myeloid Leukemia (AML)
Status: Recruiting
ClinicalTrials.gov: NCT03041688A Phase 1B Study of KRT-232 (AMG-232) in Combination With Decitabine in Acute Myeloid Leukemia.
Glioblastoma
Status: Recruiting
ClinicalTrials.gov: NCT03107780Phase 0/I Study of AMG 232 (KRT 232) Concentrations in Brain Tissue in Patients With Recurrent Glioblastoma and of AMG 232 (KRT 232) in Combination With Radiation in Patients With Newly Diagnosed Glioblastoma and Unmethylated MGMT Promoters.
Multiple Myeloma
Status: Recruiting
ClinicalTrials.gov: NCT03031730A Phase 1 Dose-Escalation and Exploratory Dose Expansion Study of KRT-232 (AMG 232) in Combination With Carfilzomib, Lenalidomide, and Dexamethasone in Relapsed and/or Refractory Myeloma.
Soft Tissue Sarcoma (STS)
Status: Active, Not Recruiting
ClinicalTrials.gov: NCT03217266A Phase Ib Trial of Neoadjuvant AMG 232 (KRT-232) Concurrent With Preoperative Radiotherapy in Wild-Type P53 Soft Tissue Sarcoma (STS)
Expanded Access Program
Expanded access, also known as “compassionate use,” refers to the use of an investigational agent outside of a clinical trial. This is a U.S. Food and Drug Administration (FDA) initiative that allows patients with serious illnesses, who have exhausted all other treatment options, the opportunity to access investigational agents that are not yet approved. It is important to remember that the safety and efficacy of these agents have not yet been established. Physicians and patients should discuss all possible benefits and risks before seeking expanded access to an investigational agent.
Kartos Therapeutics will evaluate each request for expanded access to KRT-232. All requests must be submitted to eap@kartosthera.com by a licensed healthcare professional. We will review the request promptly and respond within 48 hours.
For more information on the FDA Expanded Access program, please see the FDA website on Expanded Access, and/or talk to your healthcare provider.